电化学 Electrochemistry
In-situ monitoring of electrochemical etching
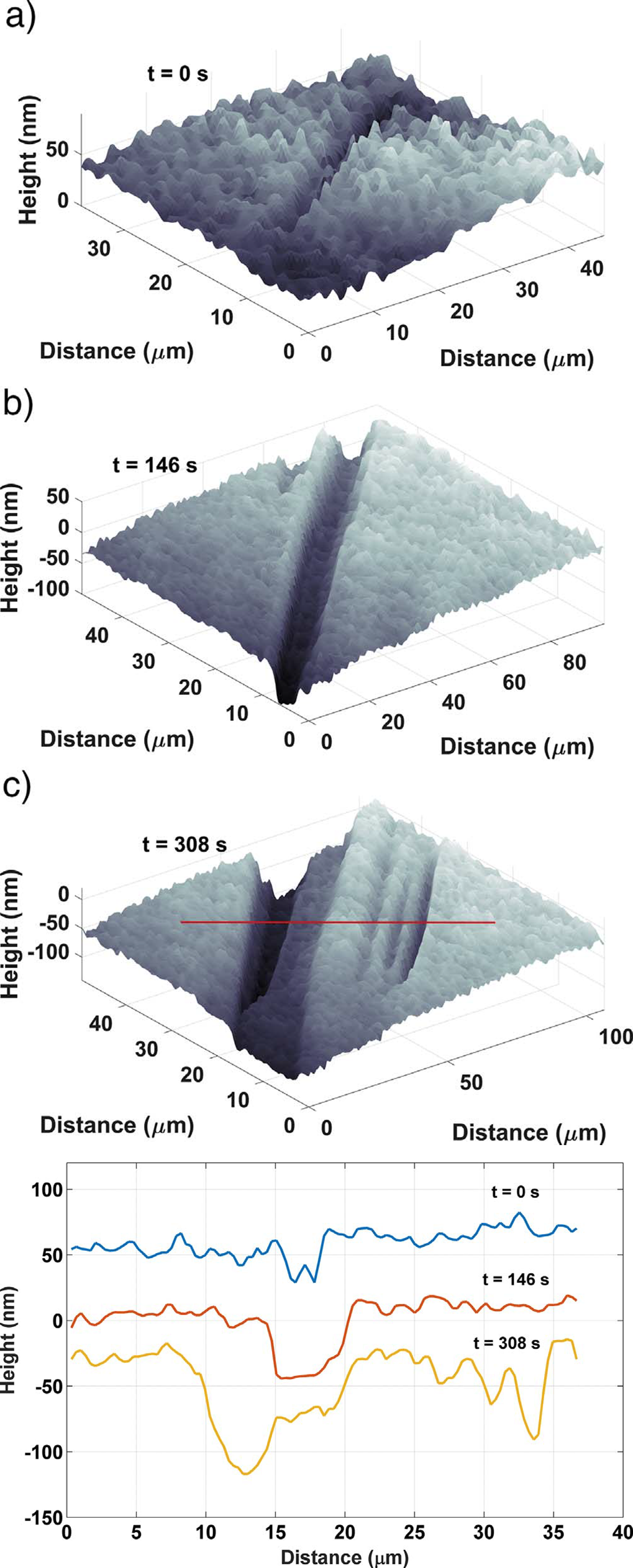
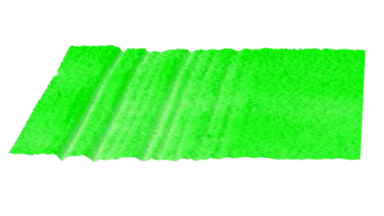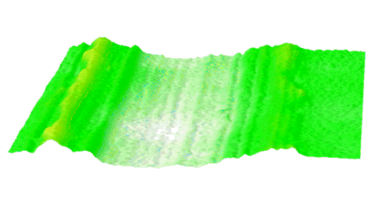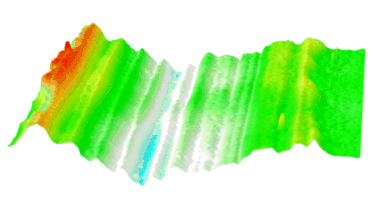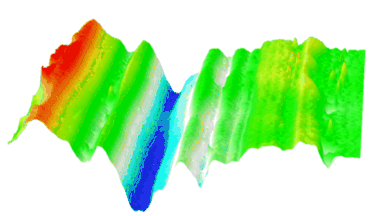
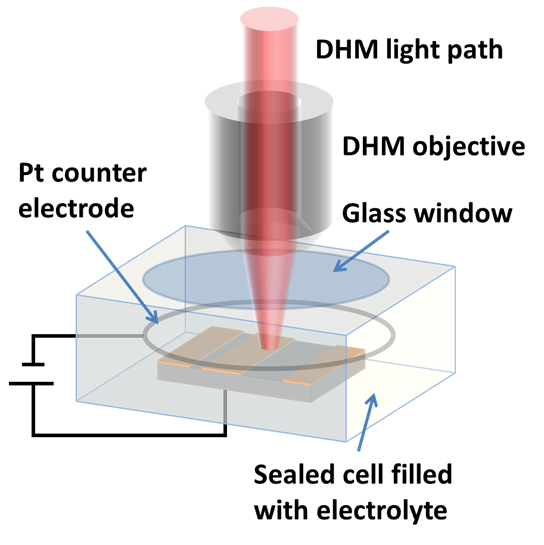
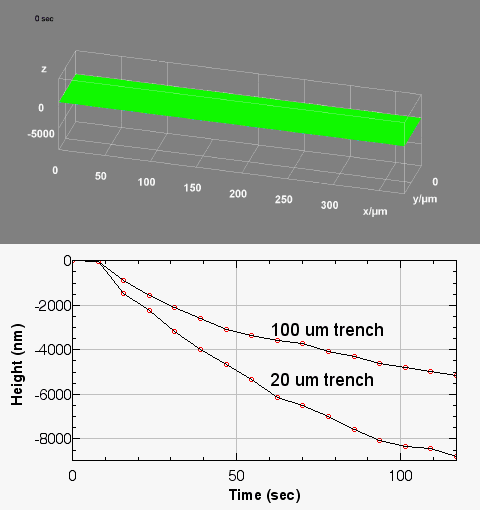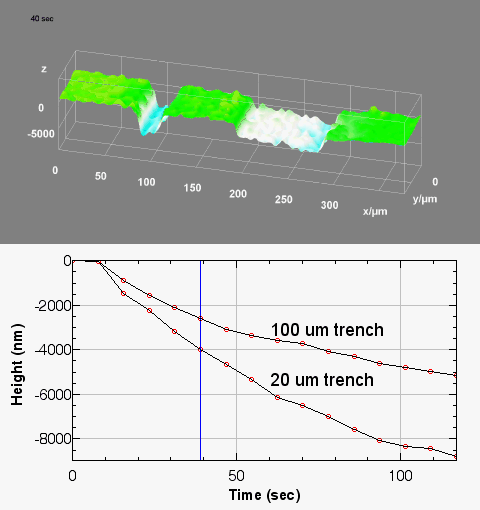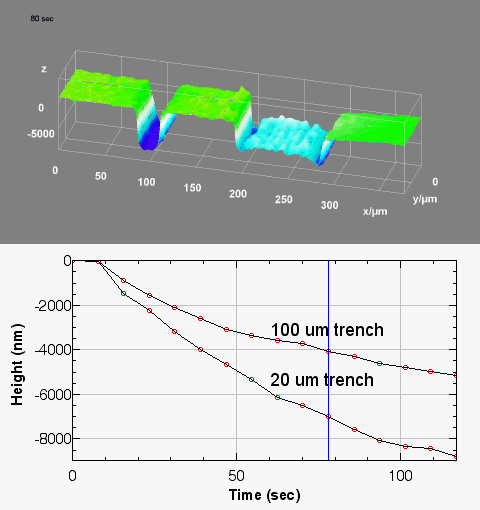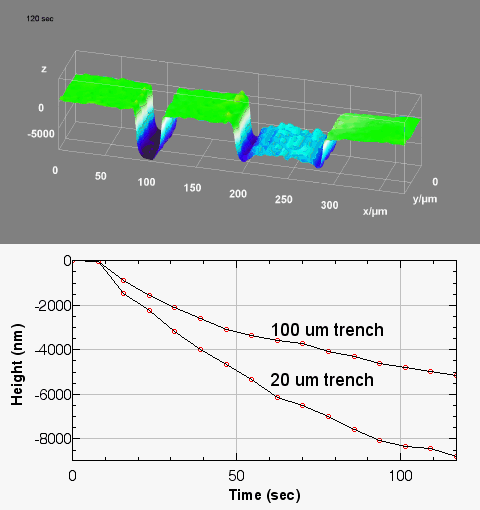
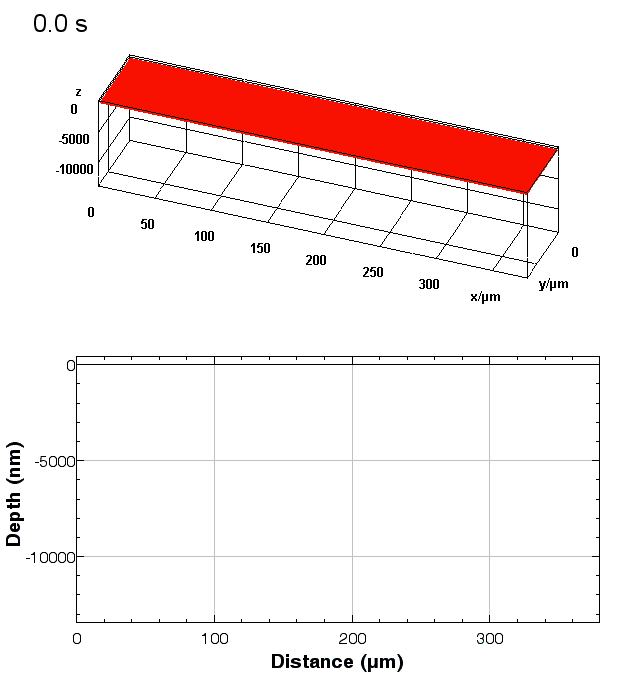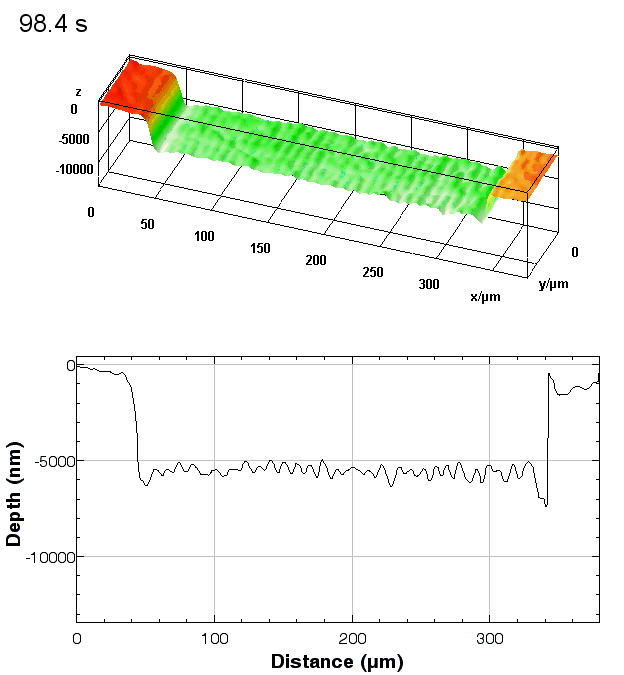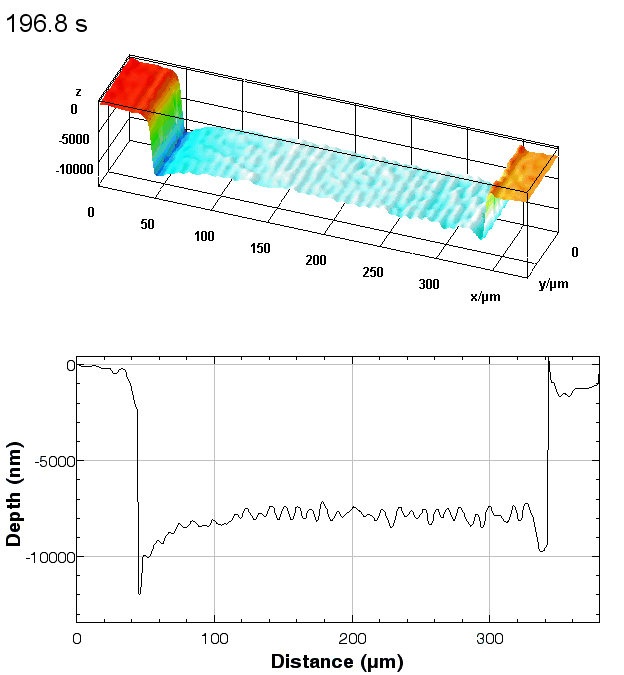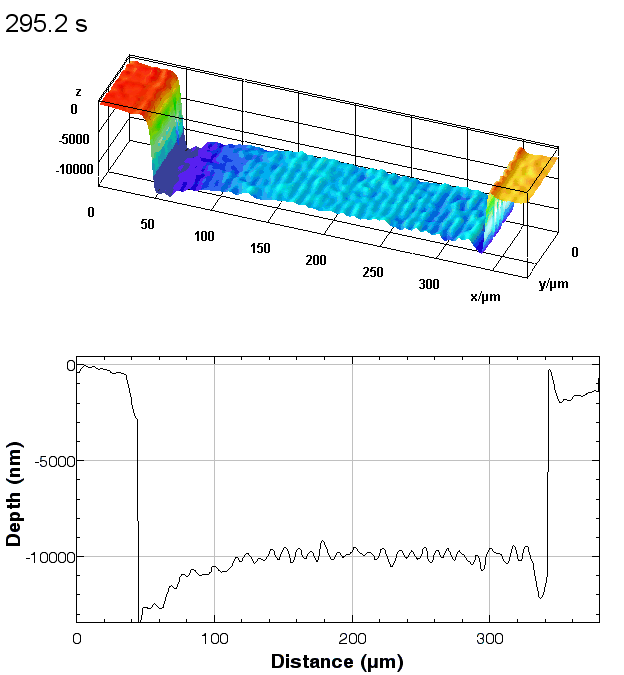
In-situ monitoring and controlling of etching processes is one of the key foundations in micro and nano structuring of materials and thin films. Conventional methods are mainly laser end point detection and optical spectrometer. Both of them monitor thickness or composition of etched layers and materials, but neither of them provide in-situ real time 3D topography measurement of the etching process with sub-micron lateral resolution. On the other hand, traditional optical and mechanical profilometers are scanning based techniques. They have static 3D topography measurement capabilities, whereas the DHM® unique non-scanning imaging technology enables to record time-sequences of instantaneous 3D topographies at camera rate with interferometric vertical resolution. Moreover, DHM® is capable of measuring through glass and liquid, allowing in-situ measurement in any environmental conditions. This application can be also extended to dry and plasma etching process.
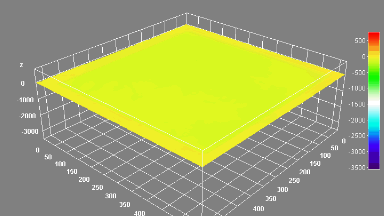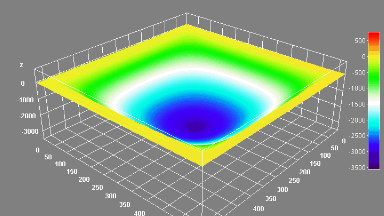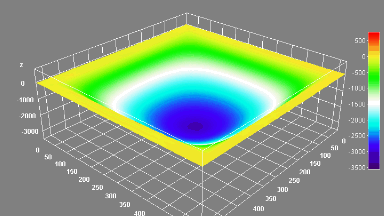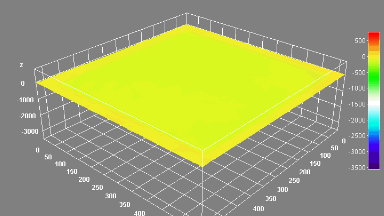
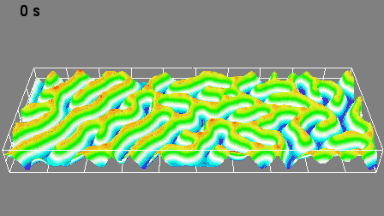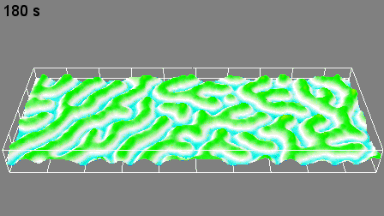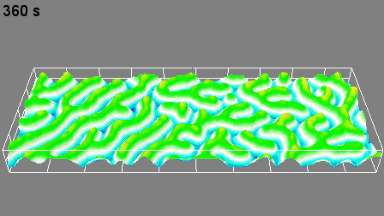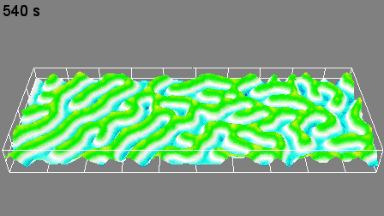
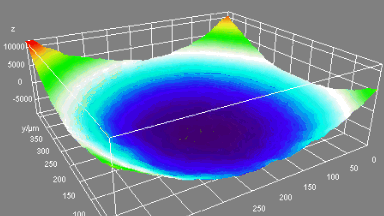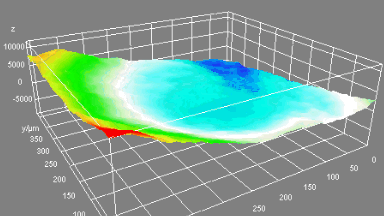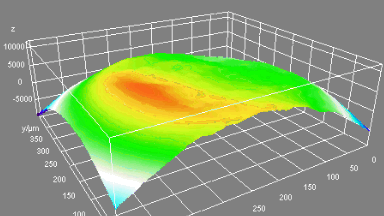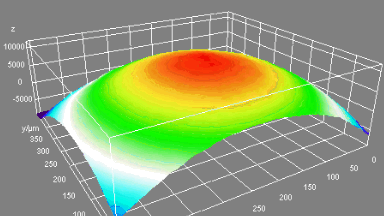
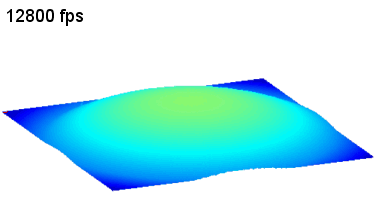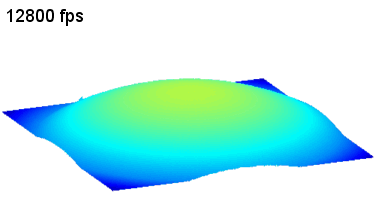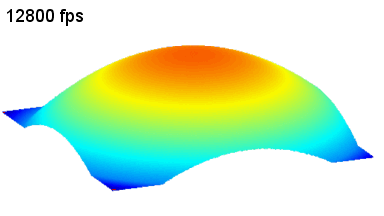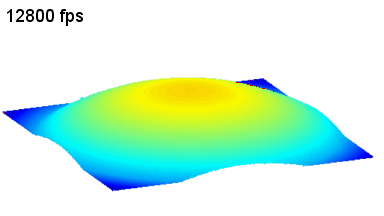
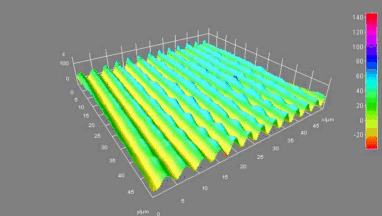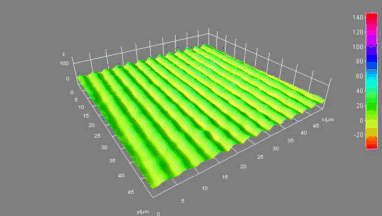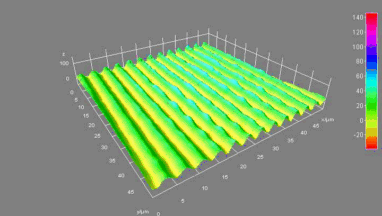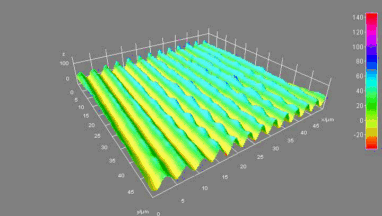
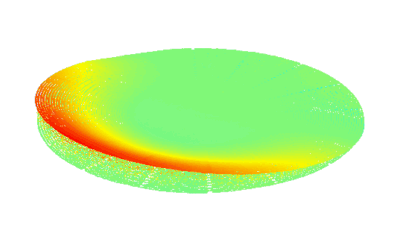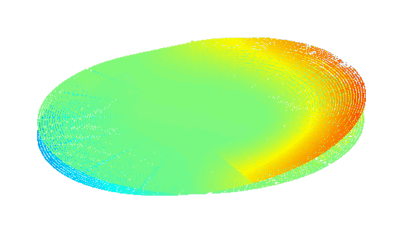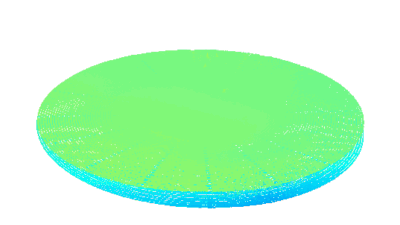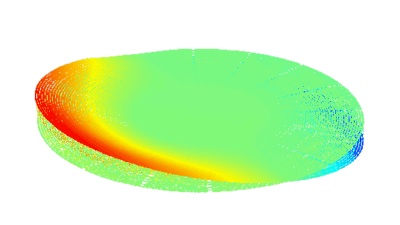
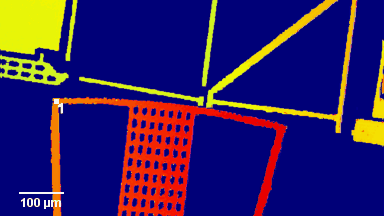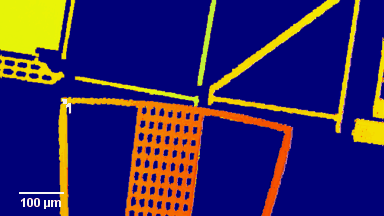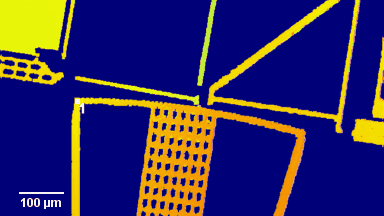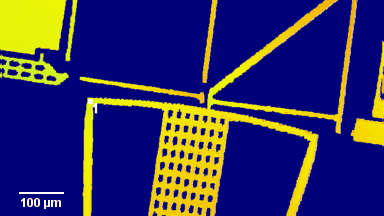
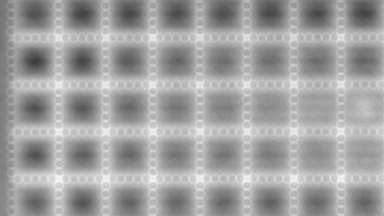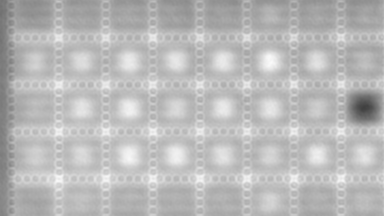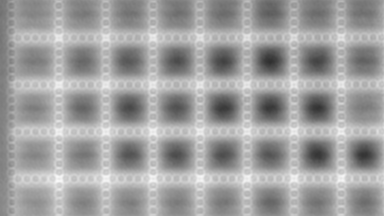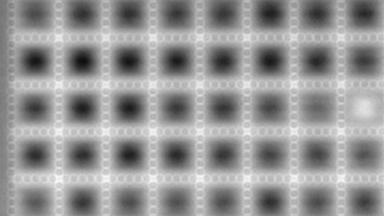
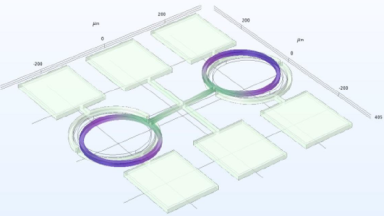
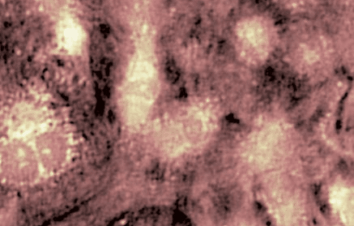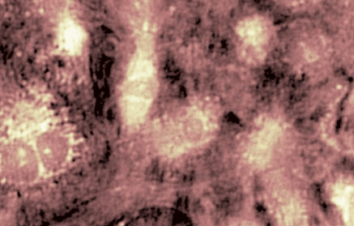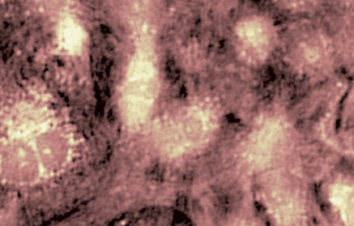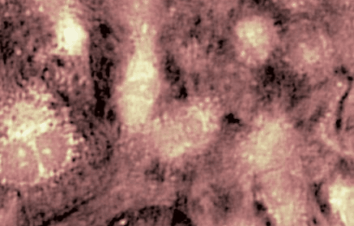
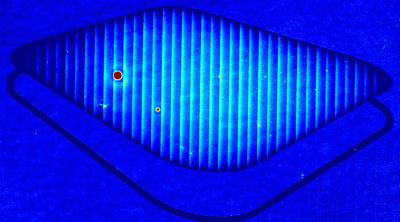
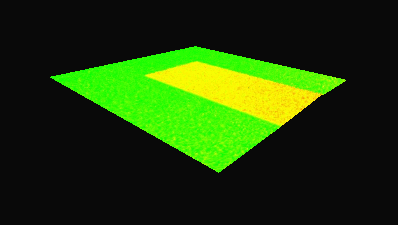
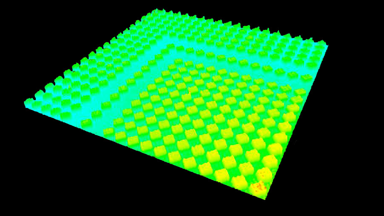
The metallic sample coated in polymer resist is patterned with trenches in different width. It is placed in liquid electrolyte and current is applied to perform electrochemical etching. The measurement is performed through the transparent window of etching chamber. Both etching depth and surface roughness can be monitored in-situ by DHM®.
Description:
- Courtesy of : Micropat SA, Switzerland
- Material: Stainless steel
- Instrument: DHM® R-2200
- Time scale: <2 minutes
- Magnification: 20x
Publication:
Laterally resolved in-situ 4D topography of electrochemical etching by Digital Holographic Microscope, Oral presentation and Proceeding of MNE2017, Braga, Portugal
Metal Electrodeposition
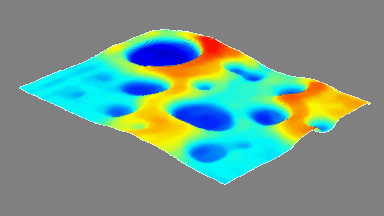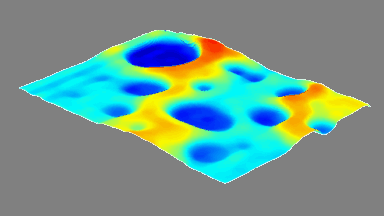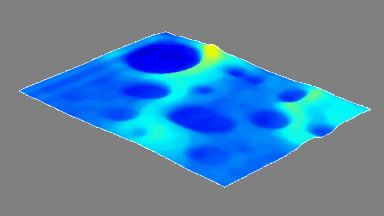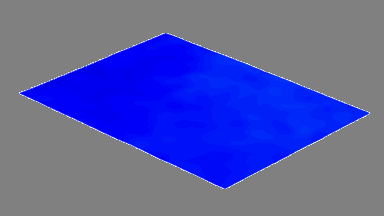
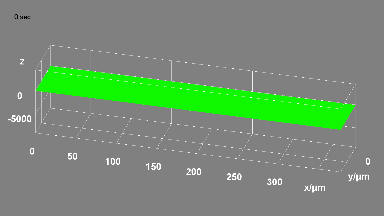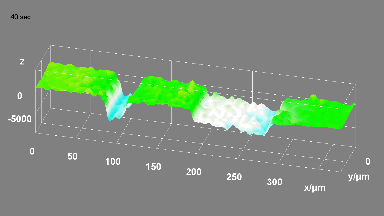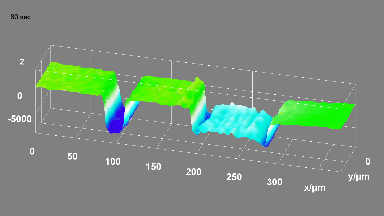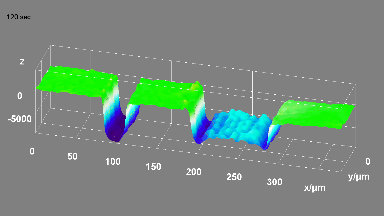
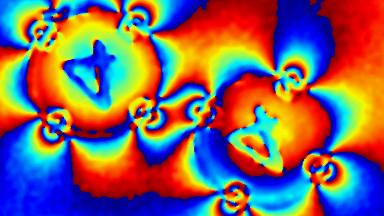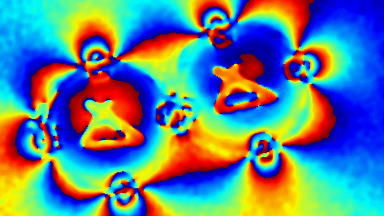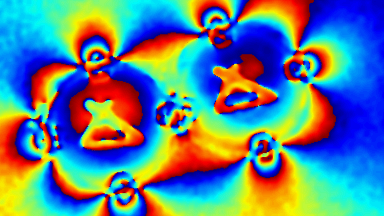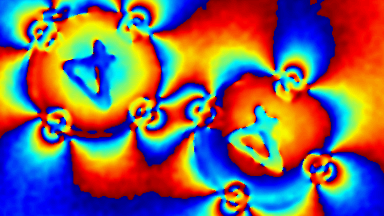
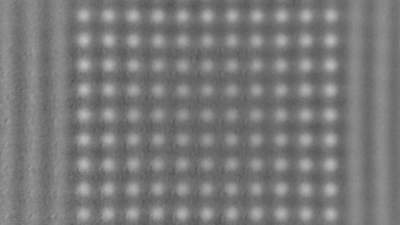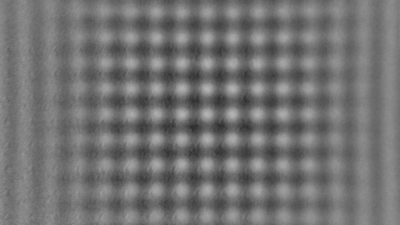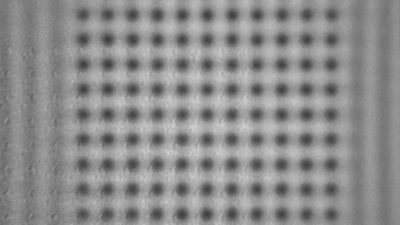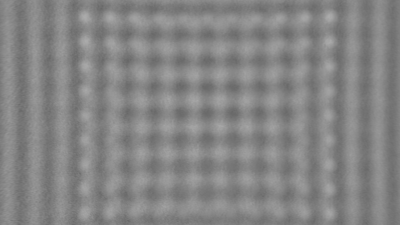
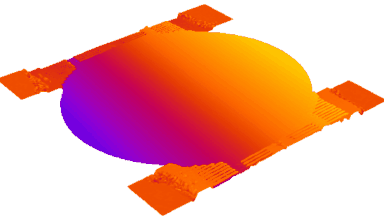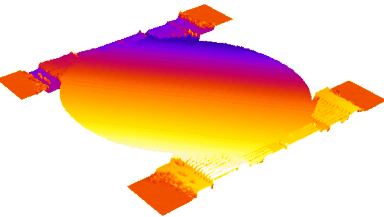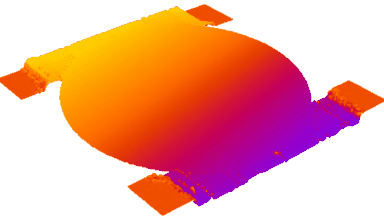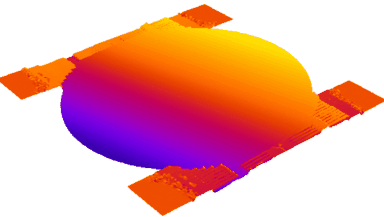
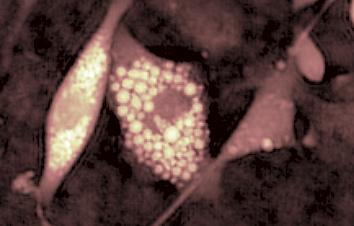
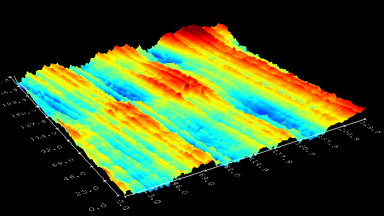
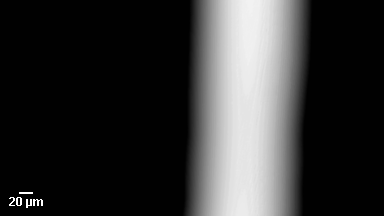
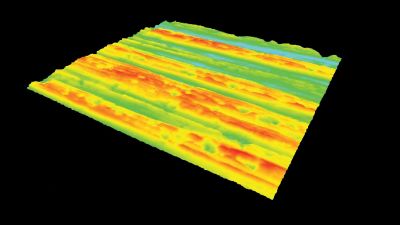
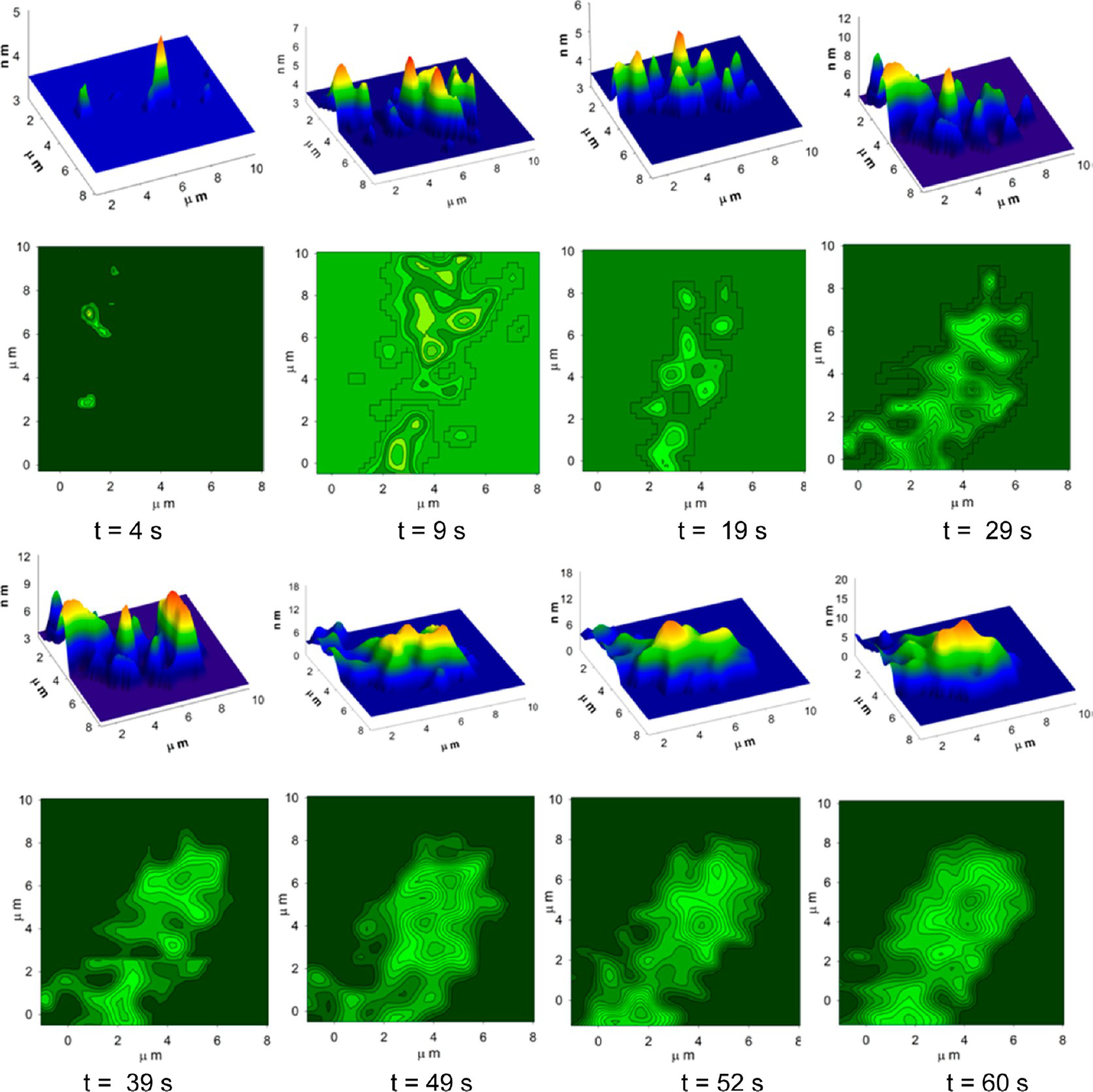
This application shows for the first time that DHM® can be used as a new analytical tool in analysis of kinetic mechanism and growth during electrolytic deposition processes. In this example, DHM® has been applied to investigate directly the electro-crystallization of a
metal on a substrate in real time (in situ) from two deep eutectic solvent (DES) systems based on mixture of choline chloride and either urea or ethylene glycol. It is demonstrated that the nucleation and growth of silver deposits in these systems are quite distinct and influenced strongly by the hydrogen bond donor of the DES.
Description:
- Courtesy of : University of Leicester, UK
- Material: Silver
- Instrument: DHM® R-1000
- Time scale: 1 minutes
- Magnification: 50x
Publication:
In Situ Electrochemical Digital Holographic Microscopy; a Study of Metal Electrodeposition in Deep Eutectic Solvents, Anal. Chem., 2013, 85 (14), pp 6653–6660 DOI: 10.1021/ac400262c
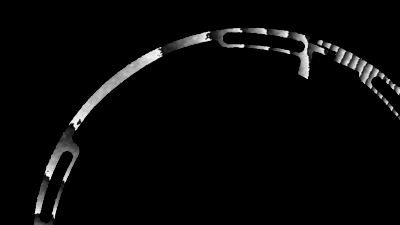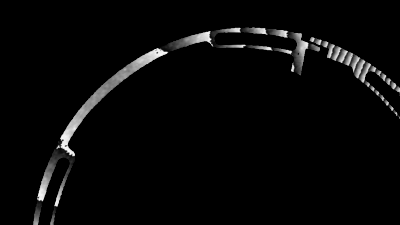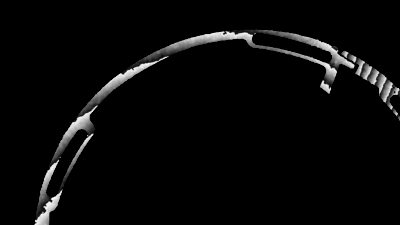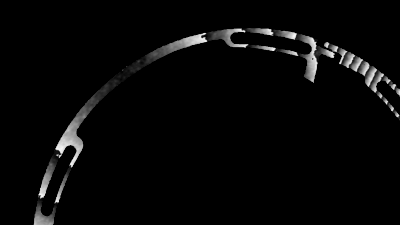
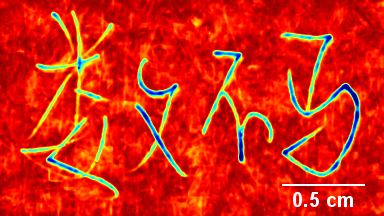
In situ observations of gypsum dissolution
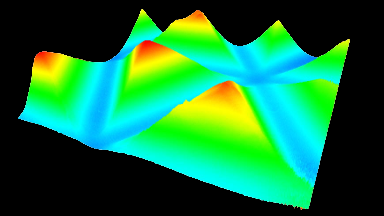
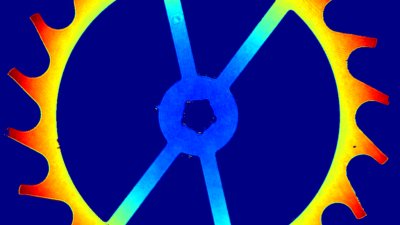
Recent topography measurements of gypsum dissolution have not reported the absolute dissolution rates, but instead focus on the rates of formation and growth of etch pits. In this example, the in situ absolute retreat rates of gypsum (010) cleavage surfaces at etch pits, at cleavage steps, and at apparently defect-free portions of the surface are measured in flowing water by reflection DHM®.
Description:
- Courtesy of : National Institute of Standards and Technology (NIST), USA
- Material: Gypsum
- Instrument: DHM® R-2200
- Time scale: 5 minutes
- Magnification: 100x
Publication:
In situ nanoscale observations of gypsum dissolution by digital holographic microscopy, Chemical Geology, Volume 460, 5 June 2017, Pages 25-36